
Grams to Moles Calculator Grams to Moles Converter
How to Convert Grams to Moles. The mole (abbreviated mol) is the SI unit of amount of substance. One mole is defined to contain exactly 6.02214076 x 10 23 elementary entities (atoms, molecules, ions or electrons). This number (known as Avogadro number) was chosen so that the mass of one mole of a chemical compound in grams is numerically equal, for most practical purposes, to the average mass.

How To Convert Moles to Grams YouTube
To convert grams to atoms first convert the mass of the element from grams to moles by dividing the given mass by its molar mass, then multiply the number of moles by Avogadro's number which is 6.02 x 10²3 atoms per mole. How do I calculate moles?

How To Convert Between Moles, Atoms, and Grams In Chemistry QUICK
An online moles to grams calculator helps you to convert moles to grams and calculate the number of atoms present in these grams. Before you go for using this free moles to grams converter, you must give a read to the article below. Let's dive into it! What Is A Mole? "Mole is basically the SI unit that represents the quantity of the substance."
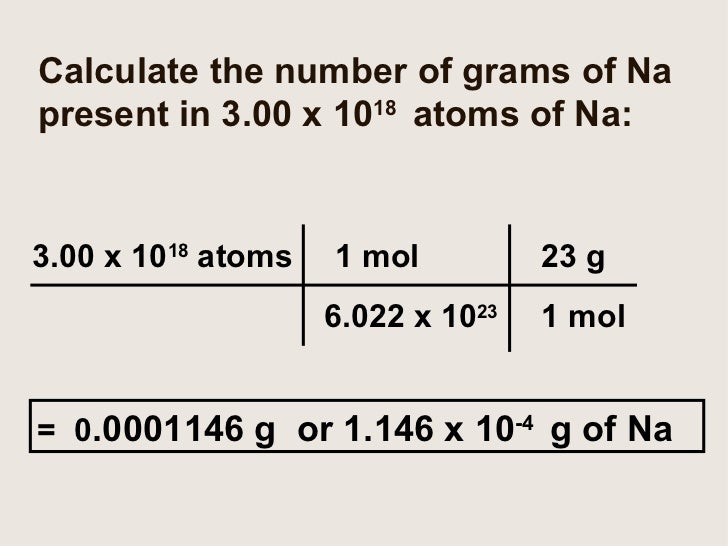
Moles To Grams Calculator slide share
The molar amount of a substance may be calculated by dividing its mass (g) by its molar mass (g/mol): The factor-label method supports this mathematical approach since the unit "g" cancels and the answer has units of "mol:". ">4.7 g K (mol K / 39.10 g) = 0.12.
.PNG)
The Mole Presentation Chemistry
This grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. In many chemistry problems, you need to convert grams to moles or moles to grams. The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles.
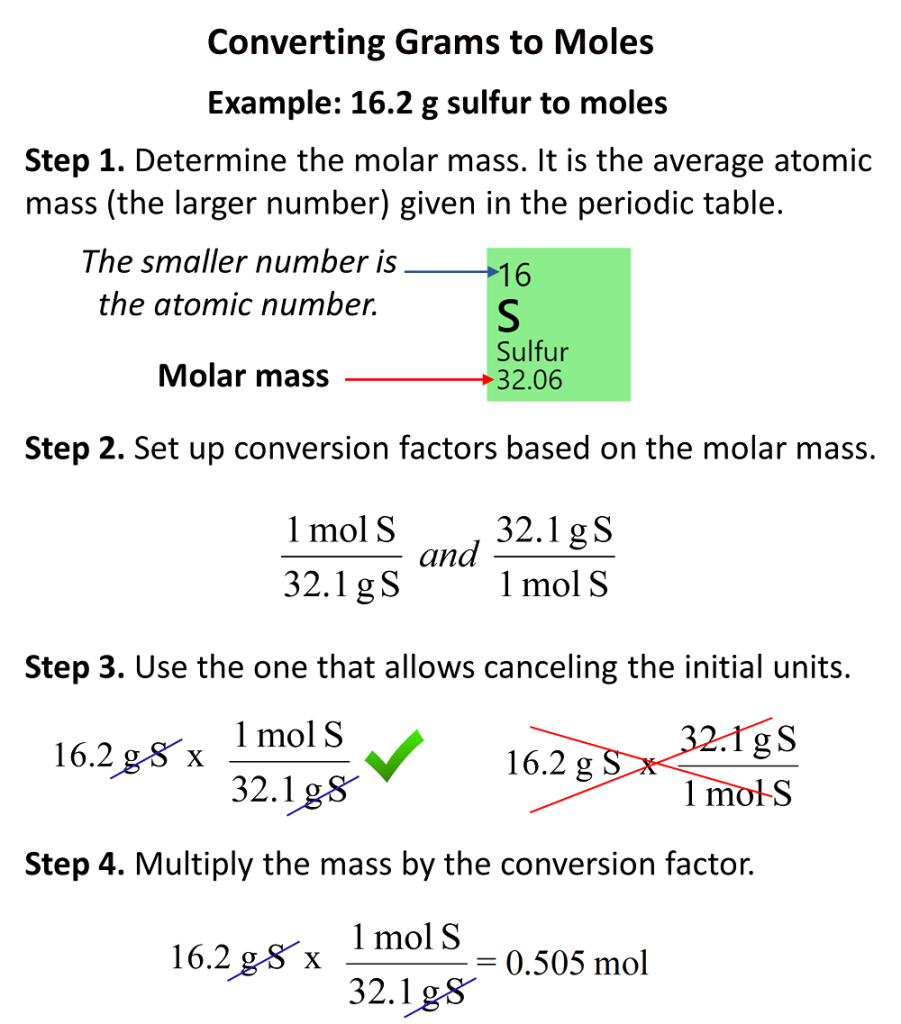
How To Convert Grams To Moles Chemistry Steps
Step 1: Enter the moles and formula weight in the input field Step 2: Now click the button "Solve" to get the conversion value Step 3: Finally, the conversion from moles to grams will be displayed in the output field What is Meant by Moles to Grams? In Chemistry, the moles to grams conversion represents the conversion of moles into grams.

How To Find Moles From Grams And Volume
Verify your answer using the calculator; What are the grams of a mole's weight? The content of a mole varies depending on what substance it is. Take your substance's atomic or molecular weight and multiply this number by how many moles you have. One mole has an atomic mass and a molecular mass. This is the same weight.

Moles to Grams Conversion Examples
This online unit converter will help you to convert the number moles to the number of grams of the atom based on the weight of the given chemical equation / formula. Moles to Grams Grams to Moles Calculator Formula Formula: Mass = Moles * Weight of Substance Example Moles to Grams If the mass is 25 moles and weight is 41 , then G = 25 * 41
How To Find Moles From Grams And Volume
Our moles to grams calculator is a useful tool for converting moles to grams and calculating the molecular weight of a compound. By understanding the relationship between moles and grams, you can easily determine the mass of a molecule in grams.
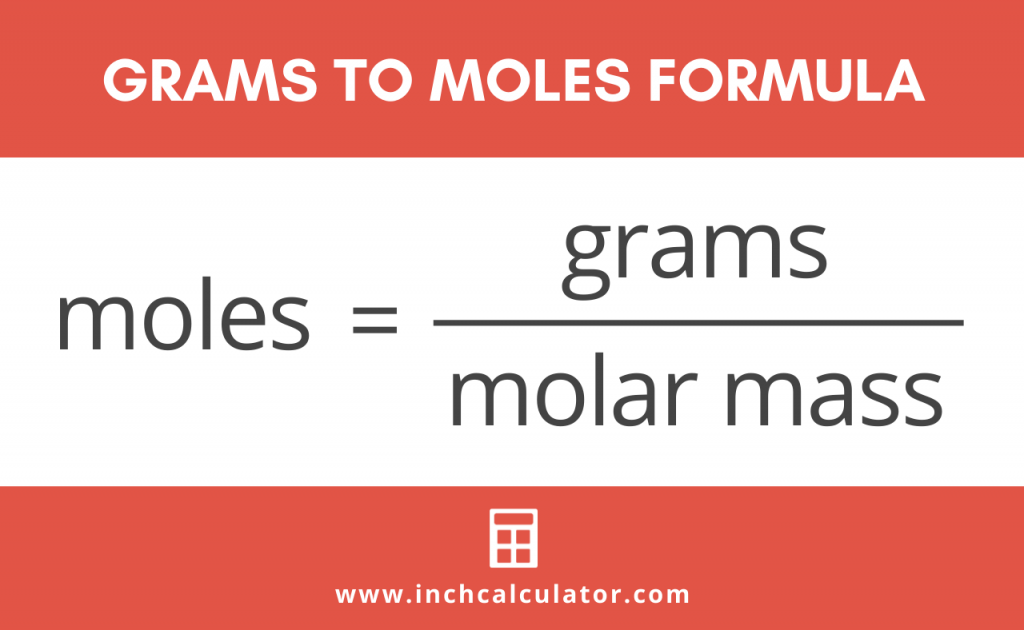
Grams to Moles Calculator Inch Calculator
This online calculator converts to moles given grams and converts to grams given moles

Moles To Grams Calculator This Nutrition
To correctly estimate the number of moles, n, of a substance of a specific mass, m, (in grams), you need to follow the grams to moles formula: n = m / M, where: M is the molar mass of this material. The unit is typically g/mol. But wait, what actually is a mole? The mole is the SI unit of measurement for the amount of a substance.
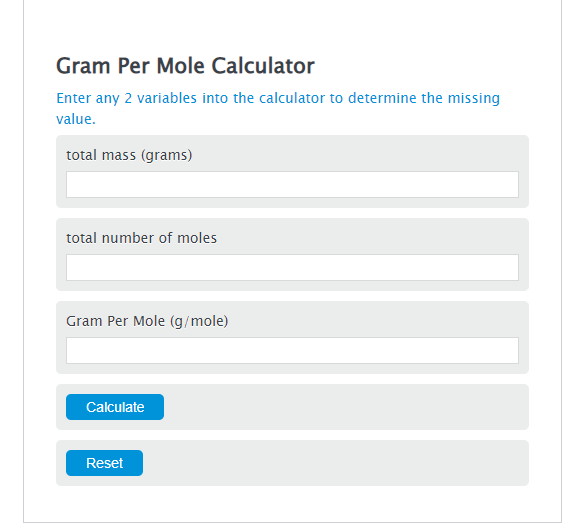
Gram Per Mole Calculator Calculator Academy
The Grams to Moles Calculator is a tool used to convert the mass of a substance in grams to the corresponding amount of moles, based on its molar mass. It aids in chemistry and chemical calculations, enabling easy conversion between mass and moles. The formula for converting grams to moles involves dividing the mass in grams by the molar mass.

How To Calculate Moles From Grams And Molar Mass
To calculate the moles from grams of a substance, we need the molar mass of the substance. The formula to calculate the moles from grams is: n = \frac {m} {m_ {\text {mol}}} n = mmolm. Where we can identify: n. n n — The number of moles, both of molecules and atoms; m.
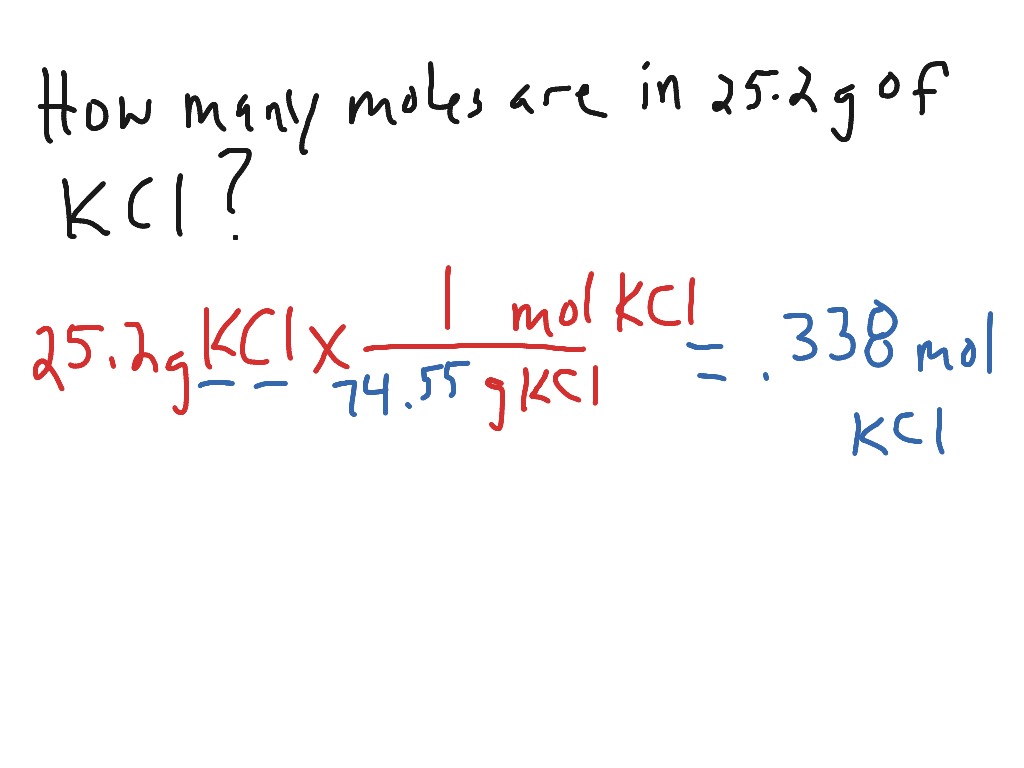
Convert grams to moles Chemistry, Science ShowMe
Use this mole calculator to find how many moles, molecular weight, and molar mass are required for your experimental measurements. What Is A Mole? The Mole is an amount unit similar to pair, dozen etc. and it contains \(6.02214076 * 10^{23}\) number of particles, whereas this number (\(6.02214076 * 10^{23}\)) is called the Avogadro's Number.

How to calculate the number of moles from grams (practice problem
1. How do I convert moles to grams easily? To convert moles to grams easily, it is suggested to use the best online calculator provided here. Enter your input number and hit on the calculate button to get the grams amount in a short span of time. 2. What is Meant by Moles to Grams? Moles to grams means the conversion of moles into grams.

How to Convert Grams to Moles 8 Steps (with Pictures) wikiHow
Moles to Gram Calculator. Moles to gram calculator converts the given data value into grams and calculates the number of atoms present in the atom. It provides a step-by-step solution to the given value. What is a mole? A mole is a unit of the amount of substance. Mole is Avogadro's number of particles, which is exactly 6.02 × 10 23. The.